Vol.01, Issue-01, July 2024
Authors
- Dr. Manish Singh Tomar, MD (Ay), MBA, CCPT, PGDYM, PGDPC
Assistant Professor, Dept. of Rasashastra & Bhaishajya Kalpana, SRS Ayurvedic Medical College, Agra, Uttar Pradesh
Director, Ayurved Bharati Hospital, Kukthari, Morena, Madhya Pradesh - Dr. Shashank Malik, BAMS
Chief Physician, Ayurhridayam Ayurvedic Clinic & Panchakarma Center, Sec 26, Noida, Uttar Pradesh
Abstract
This study evaluates the efficacy and safety of OSTYC100, a polyherbal formulation, in treating senile osteoporosis. The study was conducted as a randomized, placebo-controlled, double-blind clinical trial over six months. Fifty patients diagnosed with osteoporosis or osteopenia were enrolled and treated with OSTYC100. The results indicated significant improvements in bone mineral density (BMD) and serum calcium levels, with no significant adverse effects reported. These findings suggest that OSTYC100 is an effective and safe treatment for managing senile osteoporosis.
Keywords
Senile Osteoporosis, OSTYC100, Bone Mineral Density, Calcium Supplementation, Ayurvedic Medicine, Clinical Trial
Introduction
Osteoporosis is a metabolic bone disease characterized by low bone mass and structural deterioration of bone tissue, leading to increased bone fragility and a higher risk of fractures. Senile osteoporosis, primarily affecting individuals over the age of 75, involves decreased bone formation and a reduction in bone mineral density (BMD), particularly affecting trabecular and cortical bone. With the aging population, the management of osteoporosis has become increasingly critical. The goal of this study was to evaluate the efficacy and safety of OSTYC100, a polyherbal formulation, in improving BMD in patients with senile osteoporosis.
Materials and Methods
Study Design
This randomized, placebo-controlled, double-blind clinical trial was conducted at Ayurhridayam Ayurvedic Clinic & Panchakarma Center, Noida, Uttar Pradesh, from February 1, 2018, to August 31, 2018. Fifty patients diagnosed with osteoporosis or osteopenia based on DEXA scanning were enrolled. Patients were randomly assigned to receive either OSTYC100 or a placebo for six months.
Inclusion Criteria
- Patients aged 50 years and above
- Diagnosed with osteoporosis or osteopenia based on DEXA scanning
Exclusion Criteria
- Pregnant women, patients with congenital disorders, endocrine disorders, malignancies, and major systemic illnesses
- Patients undergoing long-term drug treatments for other conditions
Treatment Protocol
Patients in the drug group received OSTYC100 capsules, while the placebo group received identical-looking placebo capsules. Both groups were instructed to take one capsule twice daily for six months.
Monitoring and Follow-Up
Patients were monitored monthly for adverse effects, and BMD measurements and biochemical markers were reassessed at the end of six months.
Results
Patient Demographics
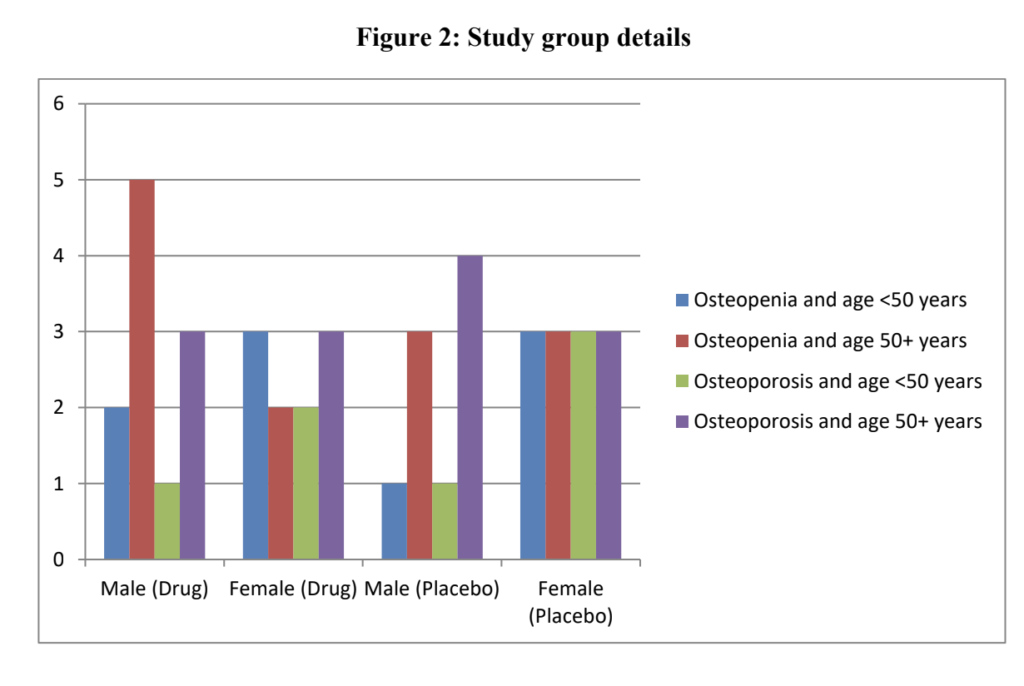
The study included 42 patients (21 in each group) who completed the six-month follow-up. A majority of the male patients were over the age of 50, while the age distribution among female patients was more balanced.
Bone Mineral Density (BMD)
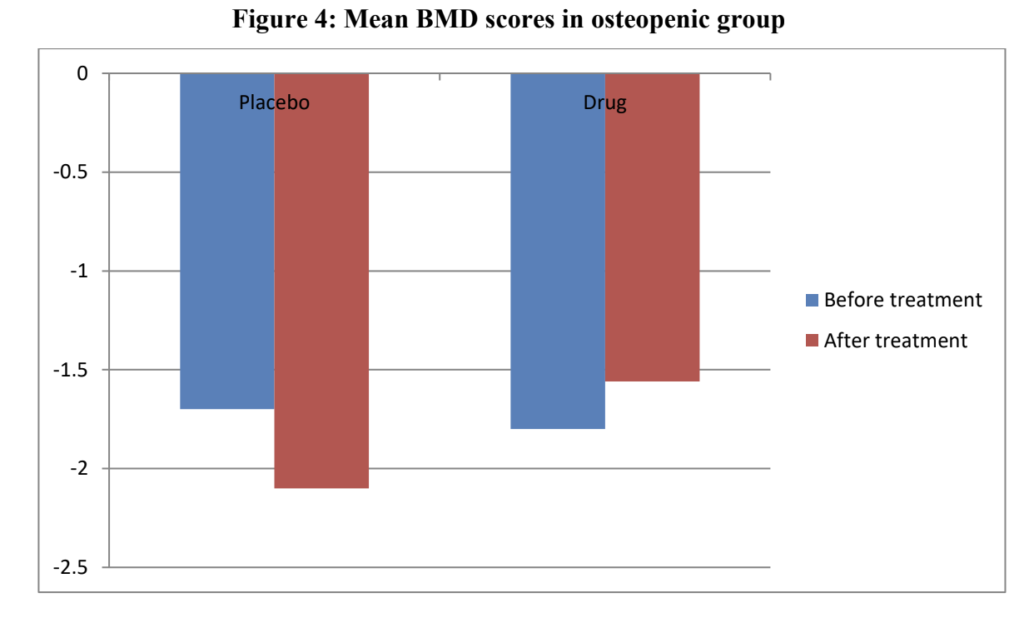
Patients treated with OSTYC100 showed significant improvements in BMD scores compared to the placebo group. The osteopenic group had a significant improvement in BMD (p=0.0497), while the osteoporotic group also showed a marked improvement (p=0.026).
Biochemical Markers
Serum calcium levels significantly increased in the osteopenic group (p=0.0435) and showed a mild, non-significant increase in the osteoporotic group. Serum phosphorus levels decreased slightly, while serum alkaline phosphatase levels showed a significant decrease in the osteopenic group (p=0.0442).
Safety and Adverse Events
No significant adverse events were reported in either the OSTYC100 or placebo groups, indicating the safety of long-term use of OSTYC100 in treating senile osteoporosis.
In both the osteoporotic and osteopenic groups, mean serum calcium level increased mildly from 7.24 ± 1.01 mg% to 8.30 ± 0.99 mg% (p=0.0656, NS), and from 8.11 ± 1.04 mg% to 9.06 ± 0.99 mg% (p=0.0435, S), respectively at the end of 6 months (Table 1).
Table_1__Mean_Serum_Calcium_Levels_Before_and_After_Treatment
| Group | Condition | Before Treatment (Mean ± SD) | After Treatment (Mean ± SD) | p value (significance) |
| OstyC100 | Osteopenia | 8.11 ± 1.04 | 9.06 ± 0.99 | p=0.0435; S |
| OstyC100 | Osteoporosis | 7.24 ± 1.01 | 8.30 ± 0.99 | p=0.0656; NS |
| Placebo | Osteopenia | 9.54 ± 2.07 | 9.25 ± 1.31 | NS |
| Placebo | Osteoporosis | 7.78 ± 1.00 | 7.89 ± 0.38 | NS |
For the mean serum phosphorus levels, the osteoporotic group showed decrease from 4.26 ± 0.64 mg% to 3.68 ± 0.96 mg%, (p=0.0595, NS); while, in the osteopenic group, mean serum phosphorus levels, decreased from 4.07 ± 0.57 mg% to 3.63 ± 0.84 mg%, (p=0.0585, NS) at the end of 6 months (Table 2). The decrease in mean serum alkaline phosphatase levels was not significant in the osteoporotic group, (p=0.0558, NS) but was significant in the osteopenic group (p=0.0442, S).
Table_2__Mean_Serum_Phosphorus_Levels_Before_and_After_Treatment
| Group | Condition | Before Treatment (Mean ± SD) | After Treatment (Mean ± SD) | p value (significance) |
| OstyC100 | Osteopenia | 4.07 ± 0.57 | 3.63 ± 0.84 | p=0.0585; NS |
| OstyC100 | Osteoporosis | 4.26 ± 0.64 | 3.68 ± 0.96 | p=0.0595; NS |
| Placebo | Osteopenia | 5.10 ± 0.47 | 4.53 ± 1.03 | NS |
| Placebo | Osteoporosis | 4.16 ± 0.64 | 3.55 ± 0.91 | NS |
Discussion
The study results indicate that OSTYC100 effectively improves BMD and related biochemical markers in patients with senile osteoporosis. The polyherbal formulation not only enhances calcium absorption but also contributes to overall bone health. The absence of significant adverse effects further supports the use of OSTYC100 as a safe, long-term treatment option for osteoporosis management.
Conclusion
OSTYC100 demonstrates significant efficacy in improving bone mineral density and related biochemical markers in patients with senile osteoporosis. Its safety profile makes it a promising option for long-term management of osteoporosis. Future studies with longer follow-up periods are recommended to confirm these findings and further evaluate the drug’s potential in bone remodeling.